While current disease-modifying therapies (DMTs) effectively reduce acute inflammation and relapses, they show limited success in addressing smouldering inflammation in chronic active lesions, a key driver of disease progression.
The Knowledge Gap:
Despite advances in MS treatment, significant challenges remain in understanding and treating chronic active lesions. Current DMTs show inconsistent results, and there is limited understanding of their effects on smouldering inflammation. Most existing studies are pharma-sponsored trials focusing on individual drugs rather than conducting comprehensive comparative analyses. The absence of standardised measurement techniques has hampered our ability to fully understand and effectively target smouldering inflammation. This gap highlights the urgent need for the accurate evaluation of this critical aspect of MS pathology.
Our Solution:
We propose a world-first international investigator-led retrospective cohort study Comparing Outcomes of Multiple Pharmacotherapies on Active Smouldering Sites in MS (COMPASS-MS). The study will evaluate the comparative effect of current DMTs on the reduction of smouldering inflammation in MS patients and will leverage our validated quantitative biomarker, Chronic Lesion Tissue Expansion (CLTE), measured through our innovative Lesion Expansion and Analysis Pipeline (LEAP).
Study Design:
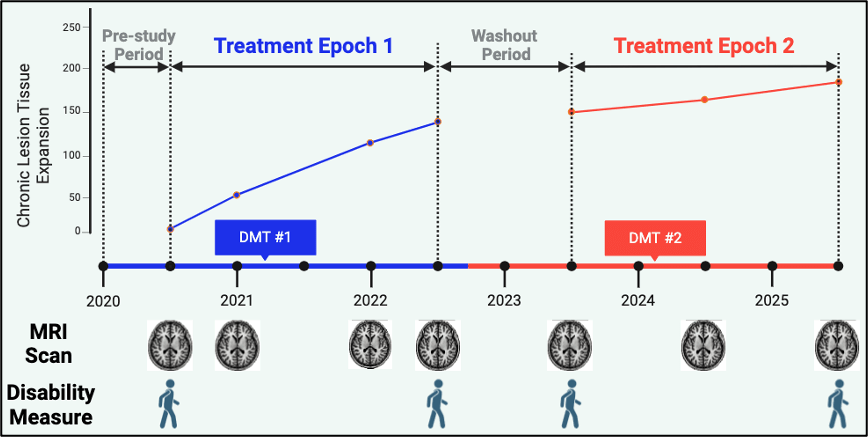
The proposed study employs a retrospective cohort design using existing patient records to assess the impact of current DMTs on smouldering inflammation in MS. Data for the study will be sourced from multiple centres, ensuring a robust and comprehensive dataset and generalisability of the results globally. The study emulates a randomised trial by comparing the degree of smouldering inflammation, brain atrophy and disability worsening among commonly used DMTs.
The study will collect clinical and imaging data from existing patient records across 4 Australian and 5 international academic hospitals and specialised MS clinics.
A key strength of COMPASS-MS lies in leveraging existing clinical databases and imaging repositories across multiple centers. This approach capitalises on comprehensive, longitudinal patient data that has been systematically collected through routine clinical care and existing research initiatives. By utilising these robust existing datasets, COMPASS-MS can generate valuable results without the extended timeframe typically required for prospective data collection, while requiring only a fraction of the costs traditionally needed for large-scale clinical trials.
Through advanced statistical modelling, COMPASS-MS is designed to emulate a randomised clinical trial of multiple DMTs in treating smouldering inflammation. This approach is particularly valuable since prospective randomised trials for large-scale comparisons are complex, lengthy, and expensive. It also means that we can get answers quickly, and those answers will be based on real-world evidence. Compared to a prospective study, where the slow process of patient recruitment and follow-up could delay results for years, COMPASS-MS is designed to deliver actionable results within 12-18 months.
Consumer and Community Involvement in Study Design:
Our approach to consumer and community involvement is rooted in extensive discussion and ongoing engagement with those directly affected by MS. The development of this proposal was significantly informed by close collaborations with over 200 MS patients and their families participating in our ongoing longitudinal study. These face-to-face encounters provided crucial insights into the daily challenges and unmet needs of those living with MS, directly shaping the focus of our research.
Complementing these individual interactions, we collaborated with Lived Experience Expert Panel (LEEP) members across Australia. LEEP, an initiative by MS Australia, is a dedicated panel consisting of individuals with personal experience with MS who provide valuable insights and guidance on research and policy. Their expertise and personal experiences with MS have been invaluable in refining our research objectives.
Expected Significance:
This multi-centre, international, investigator-led cohort study will be the first to comprehensively compare the effectiveness of current DMTs in mitigating smouldering inflammation in MS. By comparing different DMTs, the study will reveal which therapies are most effective at reducing smouldering inflammation and associated disease progression.
Funding Status and Challenges:
Despite the compelling rationale and robust study design, securing adequate funding for COMPASS-MS remains a significant challenge. Our applications to major funding bodies, including the National Health and Medical Research Council (NHMRC), have not progressed, while both MS Australia and the National MS Society (US) have indicated current funding constraints that prevent their support. Additionally, institutional funding pathways through our university have been exhausted. These funding limitations currently impede the implementation of this critical research initiative, delaying our ability to address the pressing need for evidence-based comparisons of DMT effectiveness in treating smouldering inflammation.
Based on our pioneering research in this field, designed with the involvement of an international team of world-renowned MS scientists and clinicians, and with the active contribution of people living with MS, COMPASS-MS represents a groundbreaking step forward. The translation of the results from this study have the potential to significantly advance MS treatment strategies and improve health and well-being for patients with MS.

