Chronic lesion tissue expansion (CLTE) pipeline offers a promising new approach for evaluating treatment efficacy in multiple sclerosis clinical trials of individual drugs addresing chronic inflammation. The efficiency of using CLTE as a trial endpoint is supported by its remarkably consistent nature within individual patients. our research reveals that relatively small patient cohorts can provide sufficient statistical power to detect meaningful treatment effects. For a clinical trial focusing on reducing chronic lesion expansion, our calculations show that detecting a treatment effect of 30-50% requires only 24 to 69 patients per treatment arm. This modest sample size requirement makes CLTE an attractive and feasible endpoint for clinical trials evaluating new therapies targeting smouldering inflammation.
However, patient selection requires careful consideration. Since CLTE varies significantly between individuals – from stable to extensively expanding – proper screening is essential. Trial participants must demonstrate an established trend of lesion expansion, requiring analysis of multiple compatible MRI scans during a pre-trial screening period.
Considering that a reduction of the lesion expansion slope is only likely in cases with a significant gradient of lesion expansion before the trial period, it’s essential to select patients exhibiting significant pre-trial lesion expansion. In our extensive RRMS cohort, such patients constituted about 25% (yellow dots).
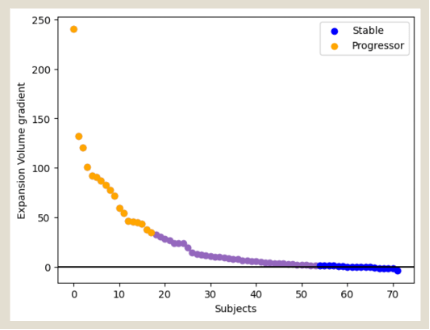
To identify patients suitable for the trial, the MRI results from the two years preceding enrolment must be examined. A minimum of three pre-trial MRI time-points are required to determine the expanding gradient. Since only 25% of patients with the highest expansion gradient are suitable for the study, the MRIs of a group four times larger must be screened.
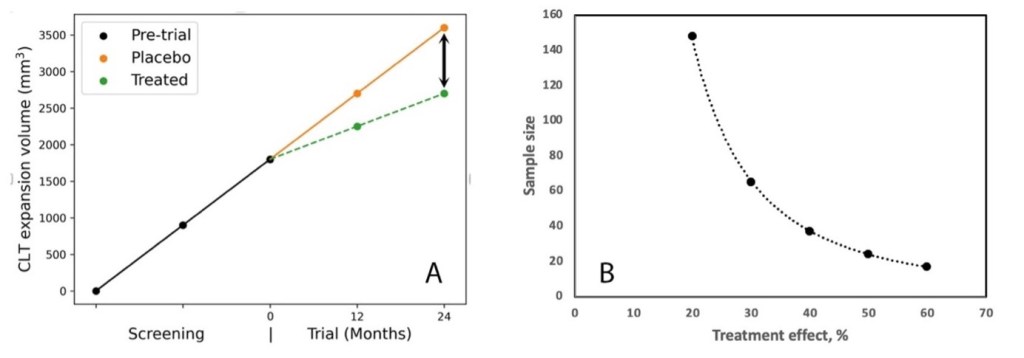
The anticipated reduction of the CLT enlargement slope in the treated group is indicative of the potential ‘treatment effect’, as shown in Fig. A below (arrow). Fig. B illustrates the sample size (per arm) required for a treatment effect ranging between 20% and 60%, which is best described by power function. For instance, to demonstrate a statistically significant (two-sided, p<0.05) reduction in lesion expansion with 80% power for a treatment effect of 50%, it would be necessary to enrol 24 patients per arm. Consequently, to meet this requirement, the pre-trial MRIs of (24x4x2 arms) = 192 patients would need to be screened.
These findings suggest that CLTE could transform how we conduct MS clinical trials. The combination of smaller required sample sizes and reliable measurement offers potential for more efficient, cost-effective trials evaluating new treatments targeting chronic inflammation in MS.
From a scientific perspective, this research will establish new standards for measuring treatment efficacy and create a framework for future comparative studies

